PUSHING THE BOUNDARIES
At Kodiak, we are working to develop disruptive technologies to prevent and treat the leading causes of blindness
ABCD PLATFORM
Our Antibody Biopolymer Conjugate Drug (ABCD) Platform is at the heart of our portfolio of next generation retinal medicines
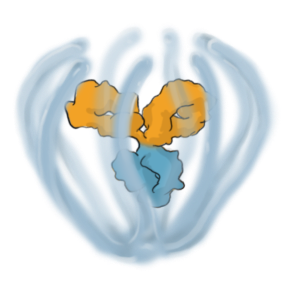

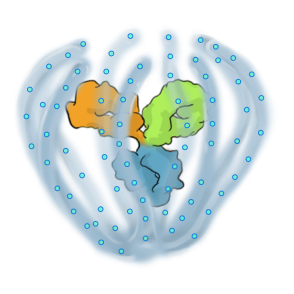
A PRODUCT PLATFORM ENABLING MULTI-MECHANISM THERAPIES EMPOWERED FOR DURABILITY
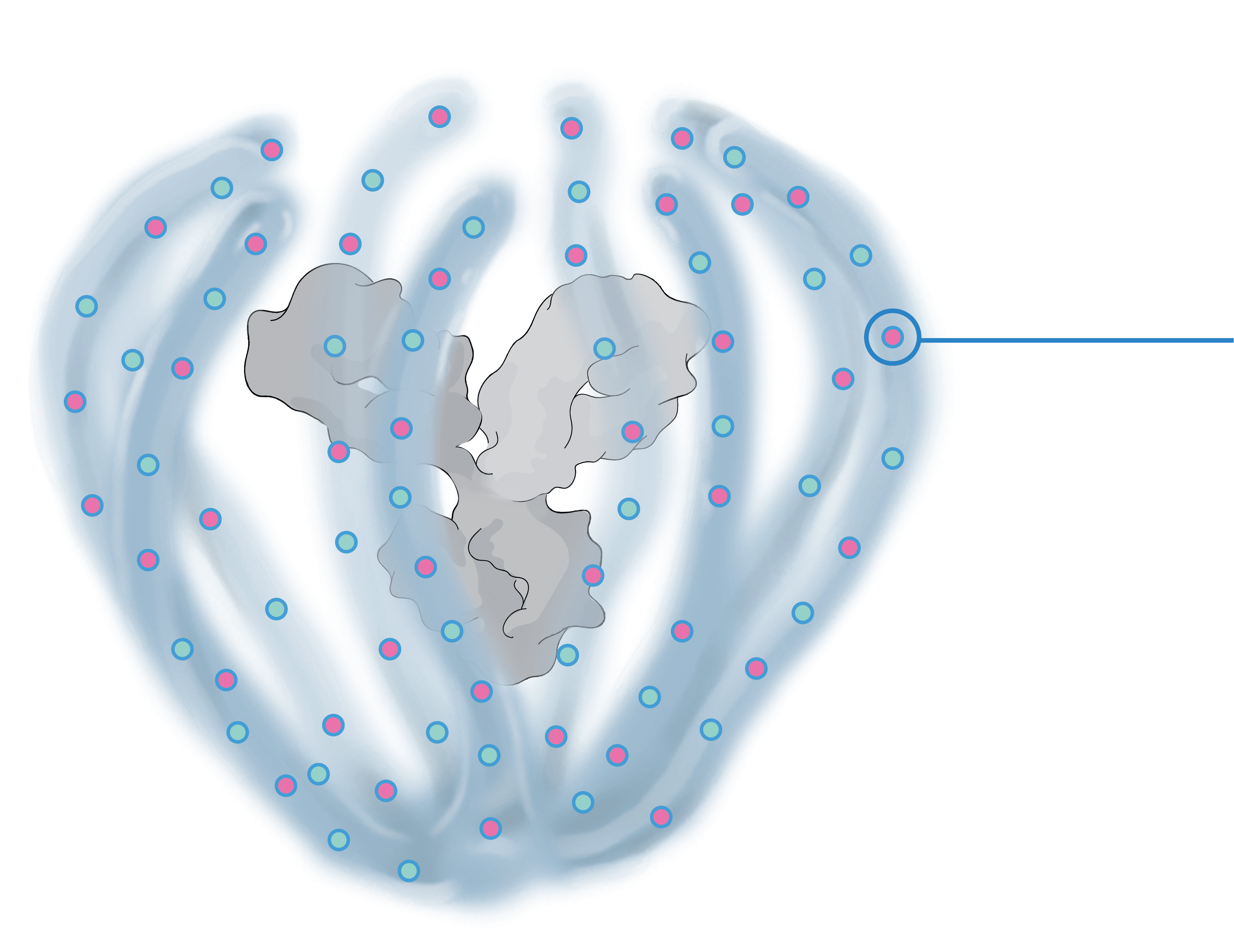
The ABCD Platform is purposefully designed for durability and powered to combine multiple modalities, such as proteins and other Active Pharmaceutical Ingredients (“API”) at a high drug-antibody ratio, in a single therapeutic
Explore the unique components of the ABCD Platform

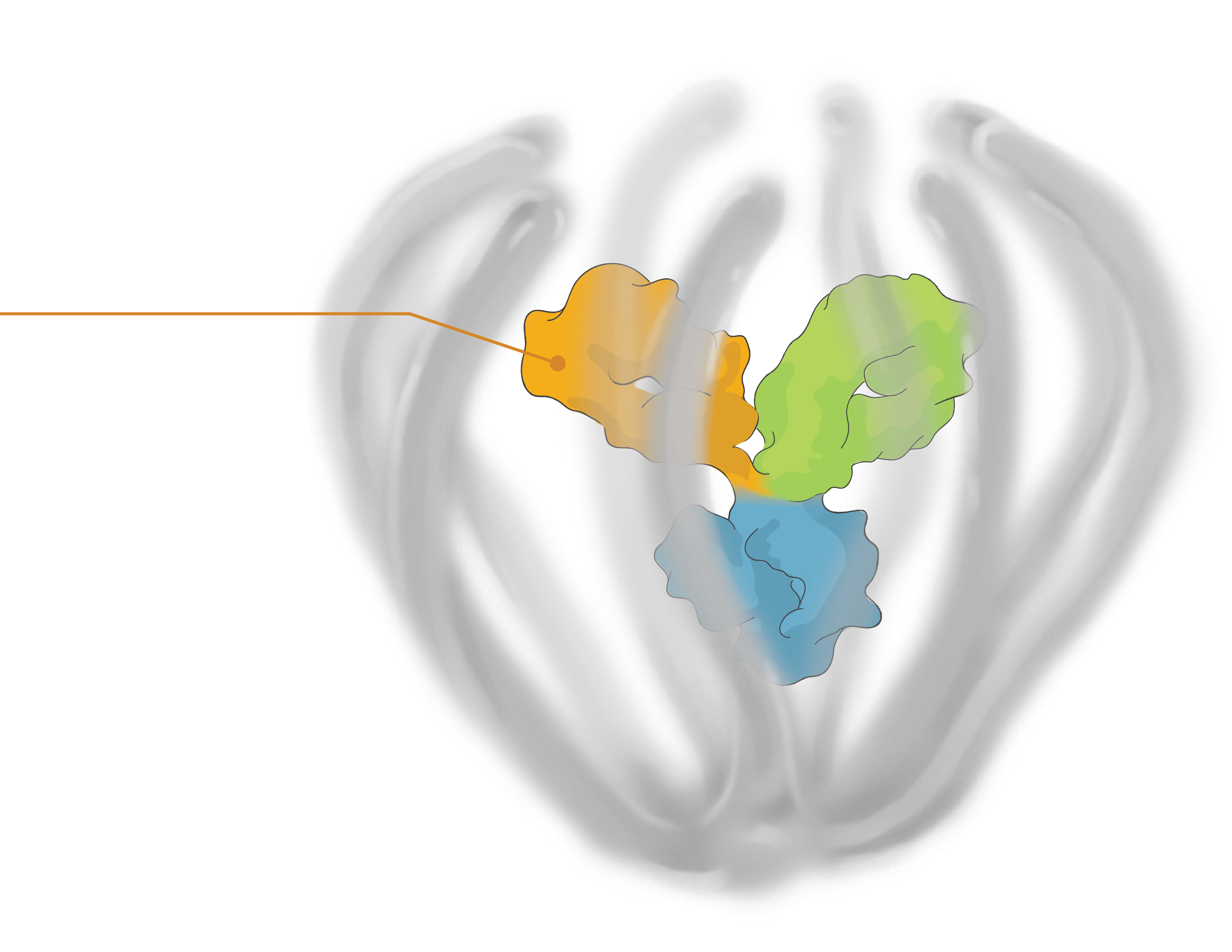
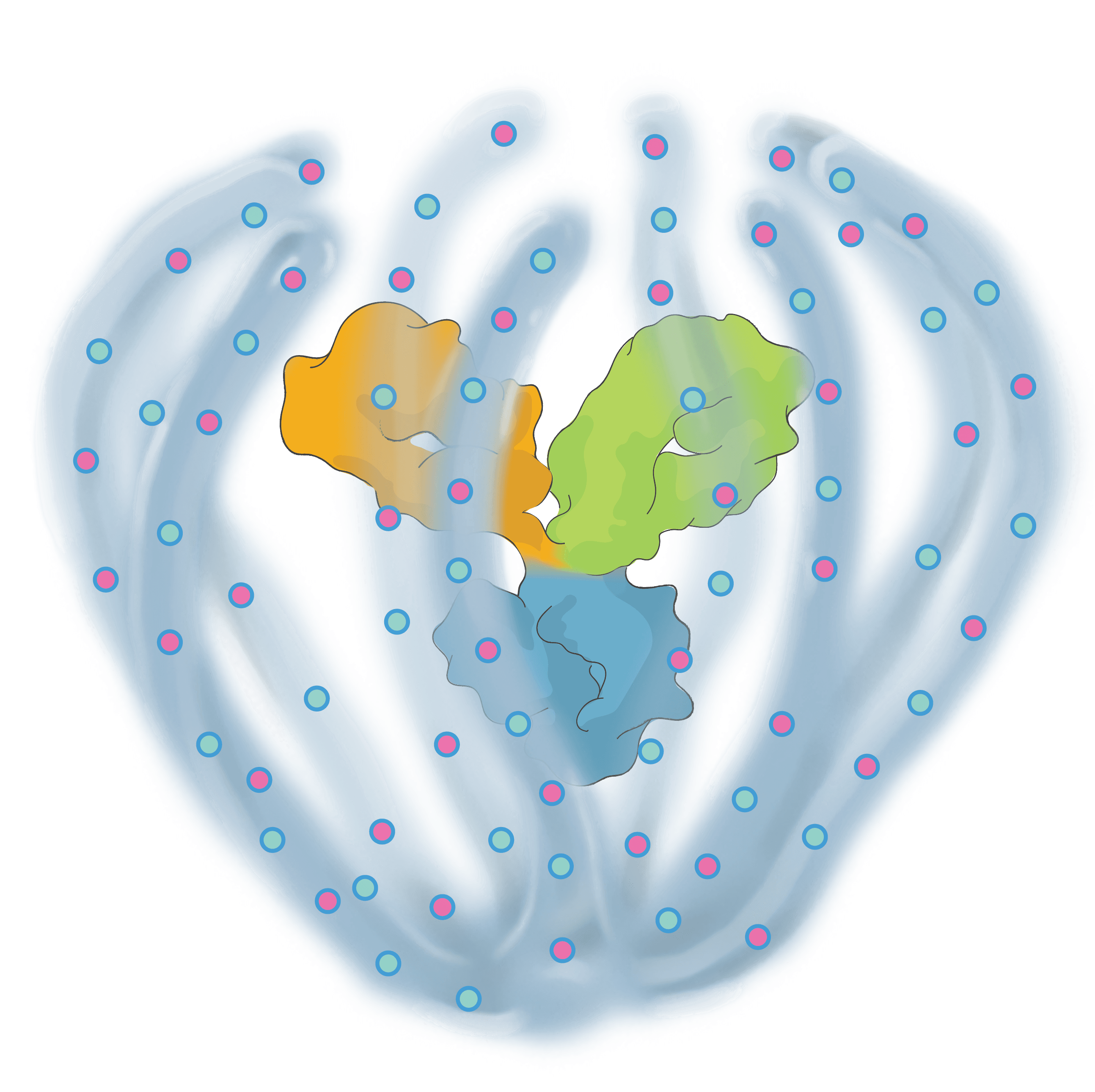
Engineered to exhibit high binding affinity and specificity. It can be any protein therapeutic, such as a monospecific or bispecific antibody.
Engineered to exhibit high binding affinity and specificity. It can be any protein therapeutic, such as a monospecific or bispecific antibody.
Conjugated to the biopolymer via a stable, site-specific linkage.
A bioinspired polymer that is engineered to make medicines last longer and extend their therapeutic benefit. It is also powered to combine multiple modalities.
A bioinspired polymer that is engineered to make medicines last longer and extend their therapeutic benefit. It is also powered to combine multiple modalities.
The biopolymer is optically clear and made of phosphorylcholine, the primary hydrophilic component of human cell membranes. It creates a water force field around the protein therapeutic without obstructing the binding sites, shielding it from non-specific interactions and providing greater on-target potency.
Small molecules and other APIs, such as oligonucleotides and peptides, can be embedded in the biopolymer backbone at a high drug-antibody ratio (DAR) and released over time to achieve targeted, multi-specific and tailored modulation of biological pathways.
Small molecules and other APIs, such as oligonucleotides and peptides, can be embedded in the biopolymer backbone at a high drug-antibody ratio (DAR) and released over time to achieve targeted, multi-specific and tailored modulation of biological pathways.
The unique combination of high DAR and tailored therapeutic benefit offers potential for broad application to multifactorial ophthalmic and systemic diseases.
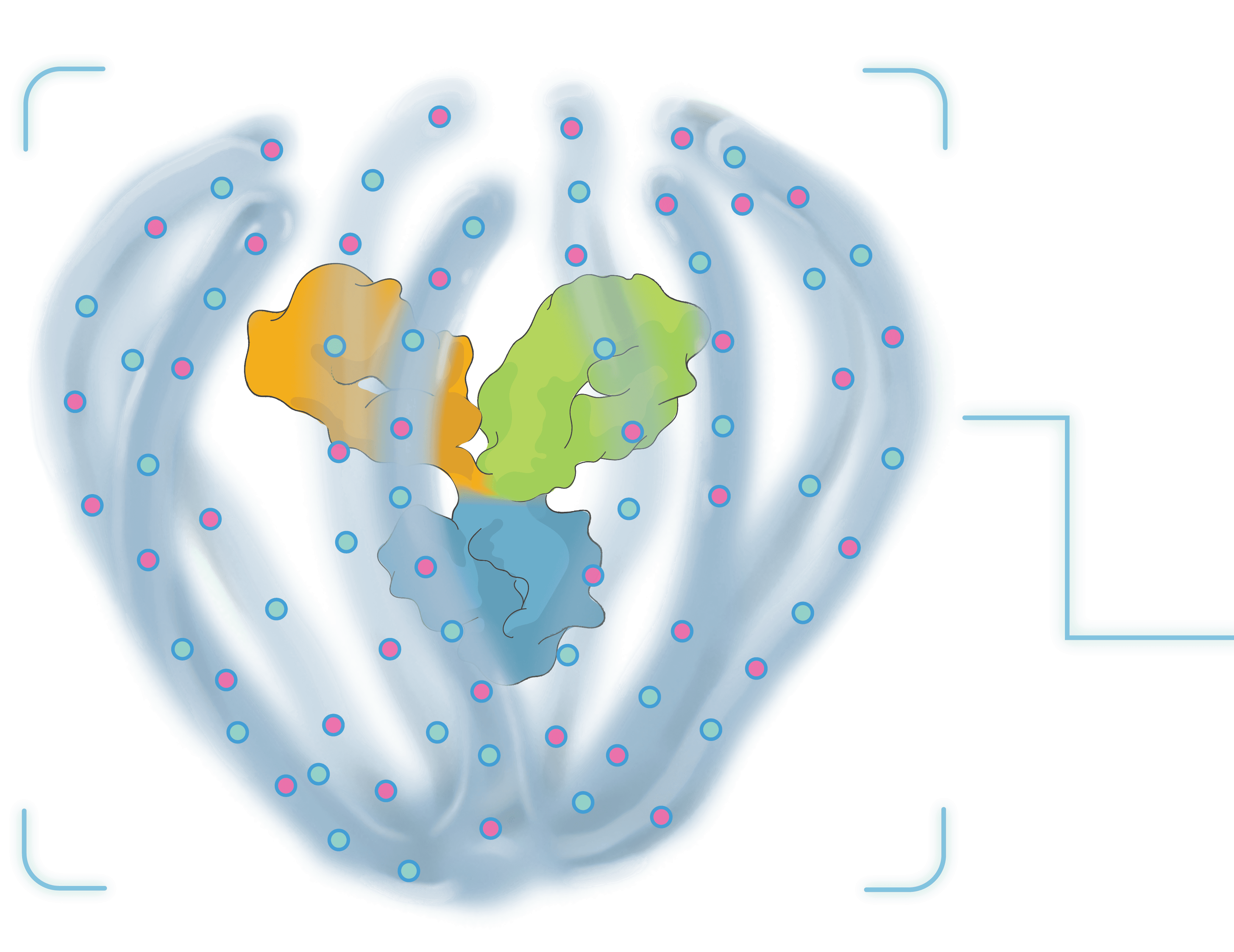
A single therapeutic purposefully designed for durability and powered to combine multiple modalities for complex ocular and systemic diseases.
More than the sum of its parts
A single therapeutic purposefully designed for durability and powered to combine multiple modalities for complex ocular and systemic diseases.
Ursus, Kodiak’s custom commercial scale manufacturing facility designed for complex antibody conjugate biotherapies, is successfully commissioned as a cGMP facility for Kodiak’s ABCD medicines.
SCIENCE OF DURABILITY
ABCD Platform-derived medicines such as tarcocimab and KSI-501 are built to last. This designed-in durability is supported by clinical data and is what we call our science of durability.
Four key elements support our science of durability


- Ocular half-life of an intravitreal biologic increases proportionally with molecular size
- The ABCD Platform leverages a high molecular weight, phosphorylcholine-based biopolymer that enables an extended ocular residence time compared to today's intravitreal biologics
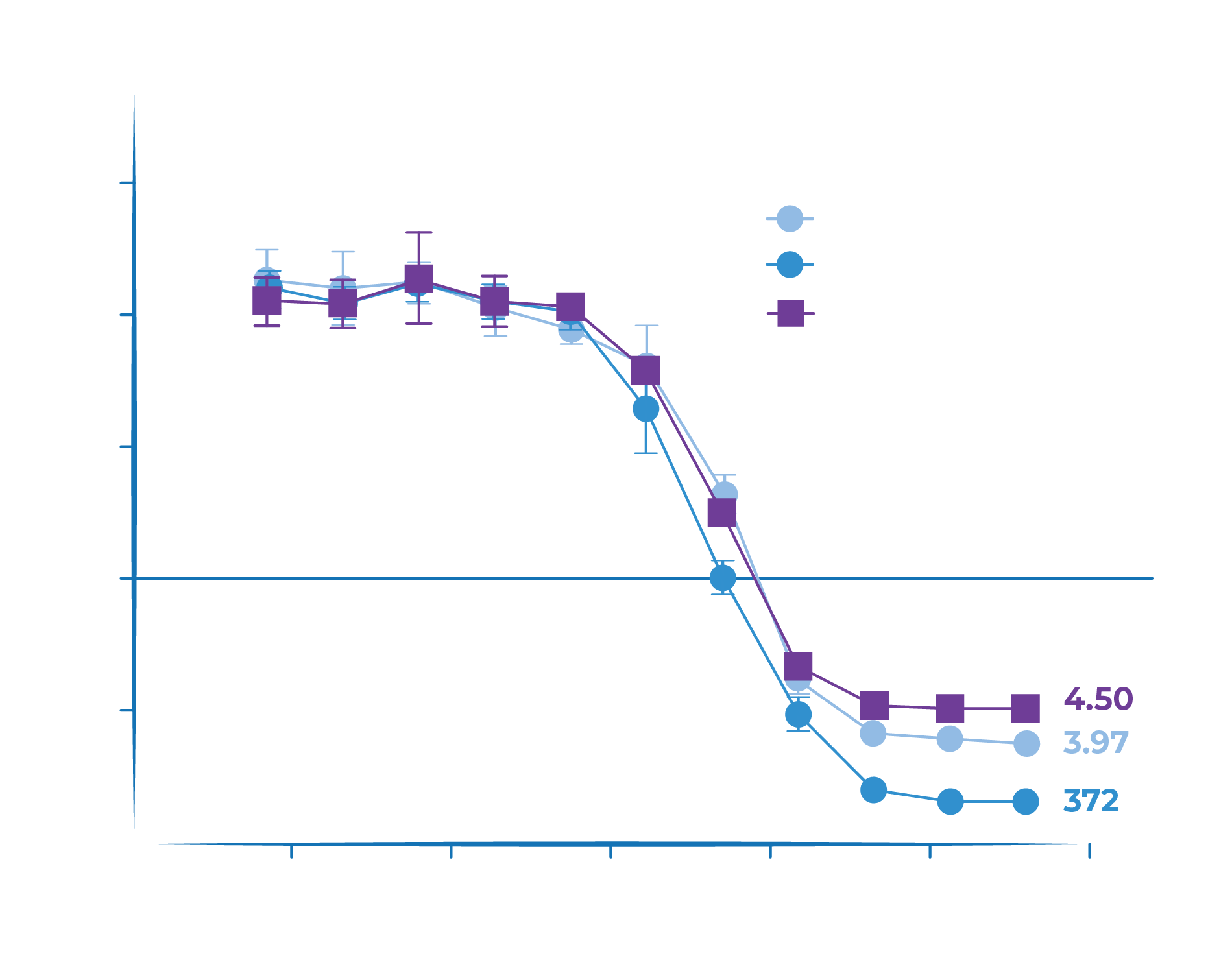
- Tarcocimab unconjugated protein and conjugated protein demonstrate high binding affinity and potency in preclinical assays – similar as aflibercept
- The increased molecular size from conjugation to the biopolymer does not impact binding affinity or potency
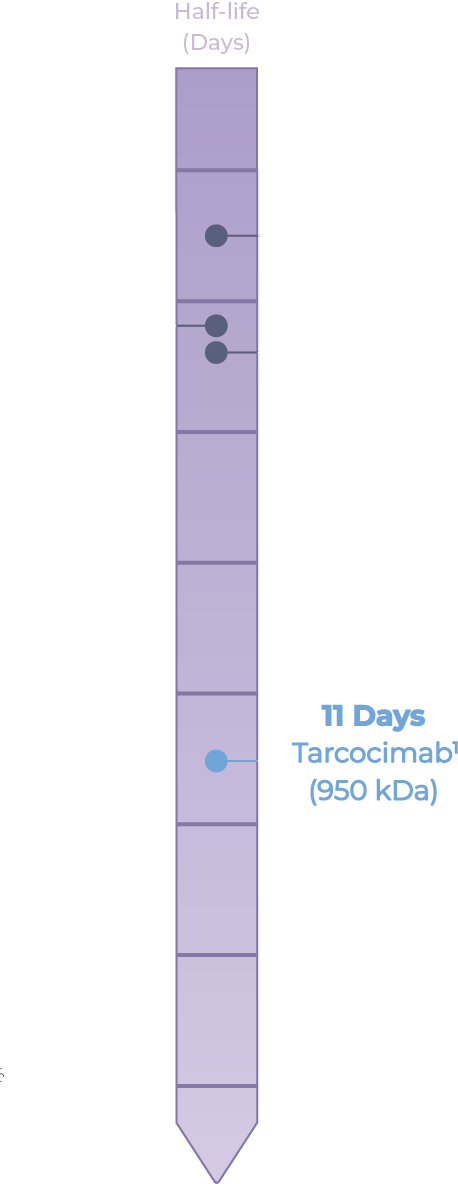
- Kodiak Data on File. Ocular half life was determined from a single 50 µL intravitreal injection of 0.725 mg of tarcocimab (conjugate) in rabbits.
- Gaudreault. et al. Retina 2007. 27: 1260-1266.
- Park S]. et al. IOVS 2016. 57: 2612-2617.
- Pharmacology / Toxicology BLA Review and Evaluation
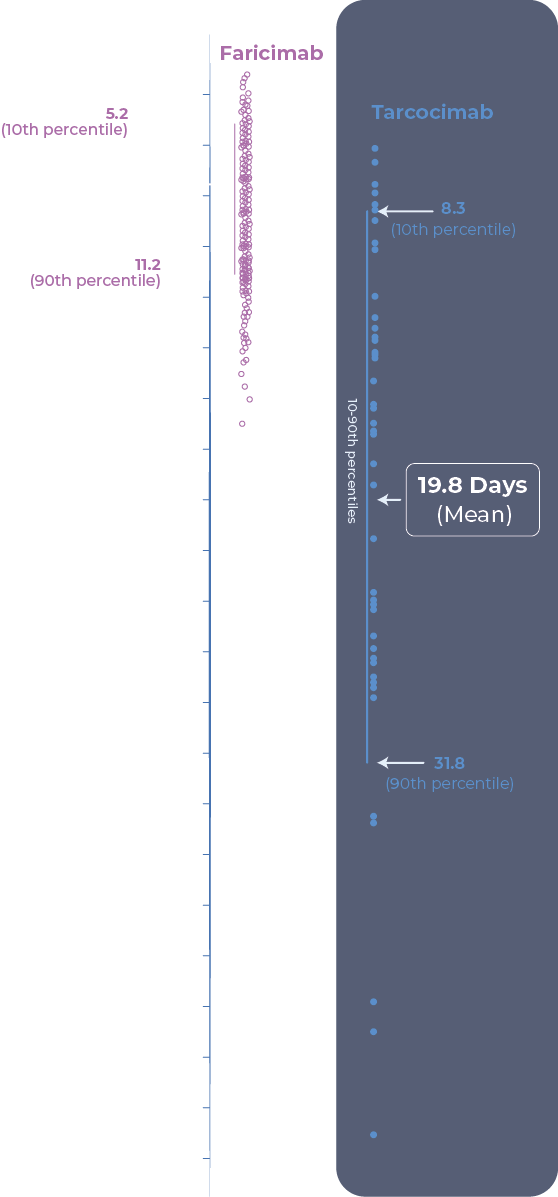
- Ocular half-life was determined from aqueous humor concentration of tarcocimab over time in the Phase 1b study in wet AMD, DME and RVO. N=47 patients, all received an intravitreal injection of 5 mg tarcocimab clinical formulation on day 1.
- VABYSMOTM (faricimab solution for injection) Prescribing Information. South San Francisco, USA: Genentech, Inc. PK and ER of faricimab, Report # 1105763
Tarcocimab demonstrated 5 to 6-month predominant durability in pivotal trials across all high-prevelence retinal vascular diseases
SEE THE ABCD PLATFORM IN ACTION
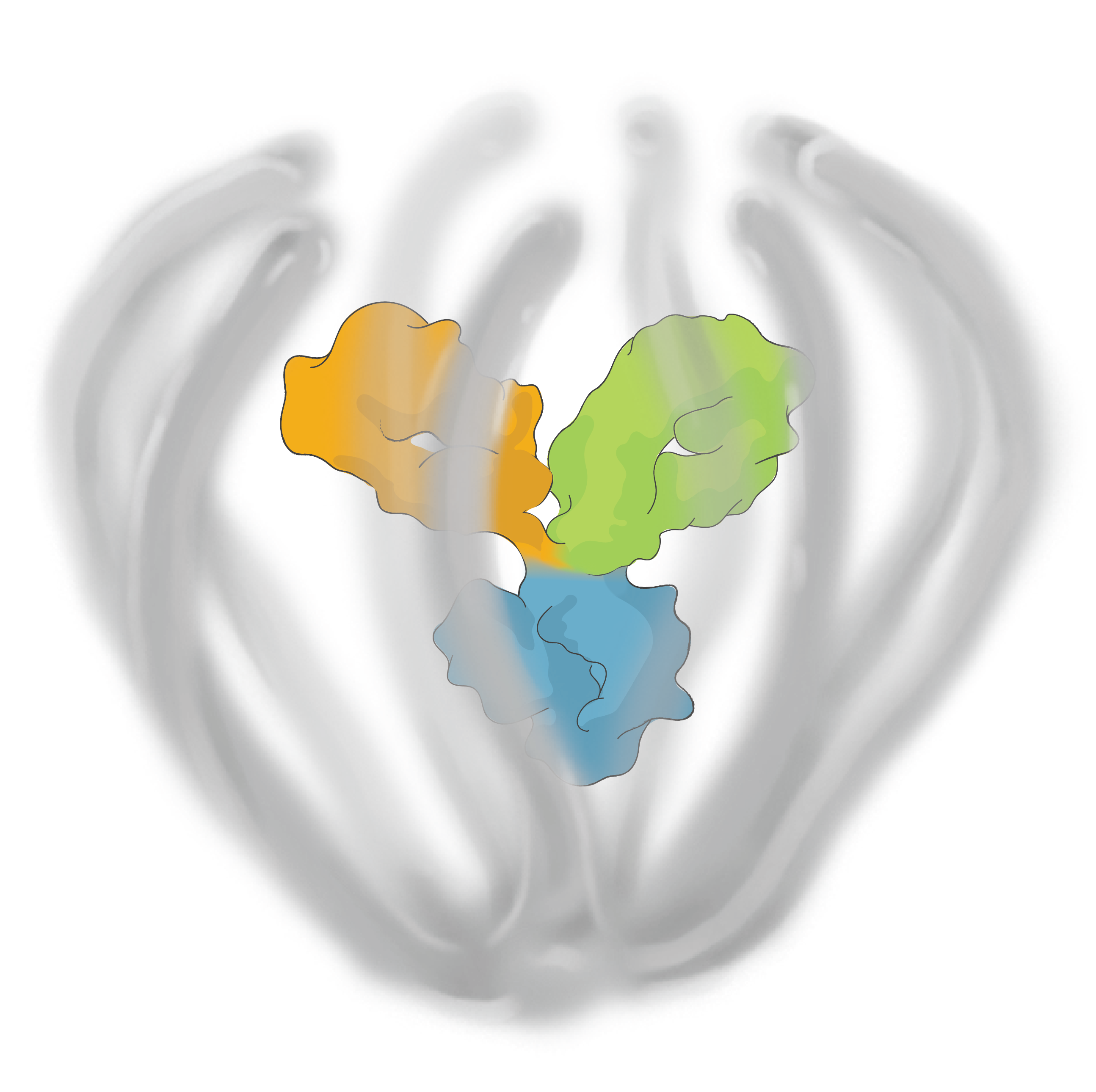
The ABCD Platform is inspired by nature and designed with water in mind. Travel through the eye to see how ABCD medicines are engineered for increased durability and efficacy

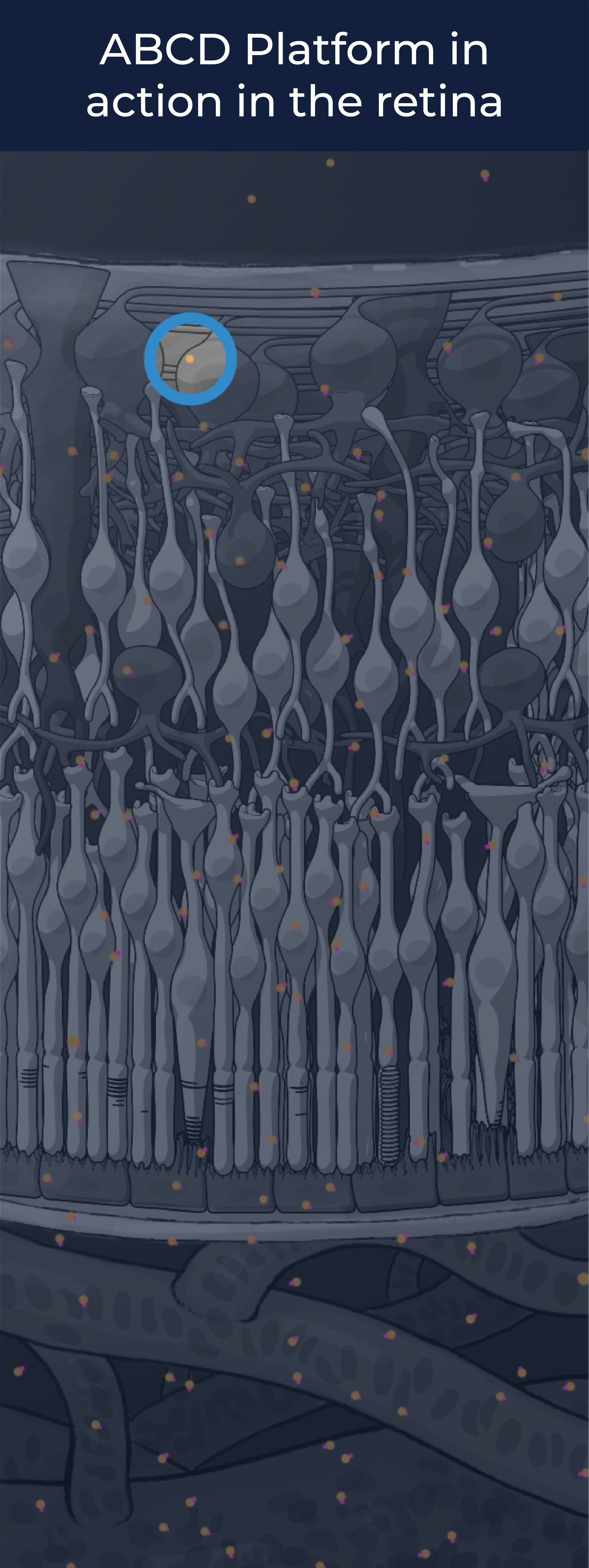
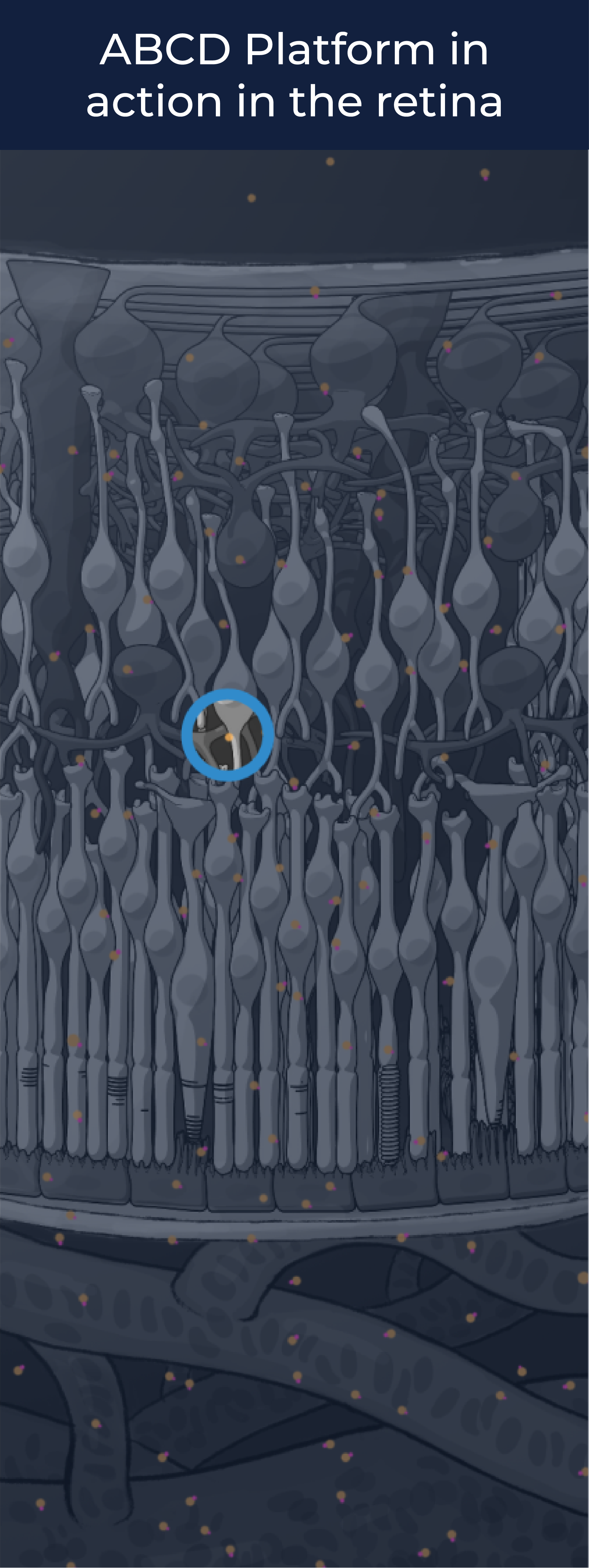
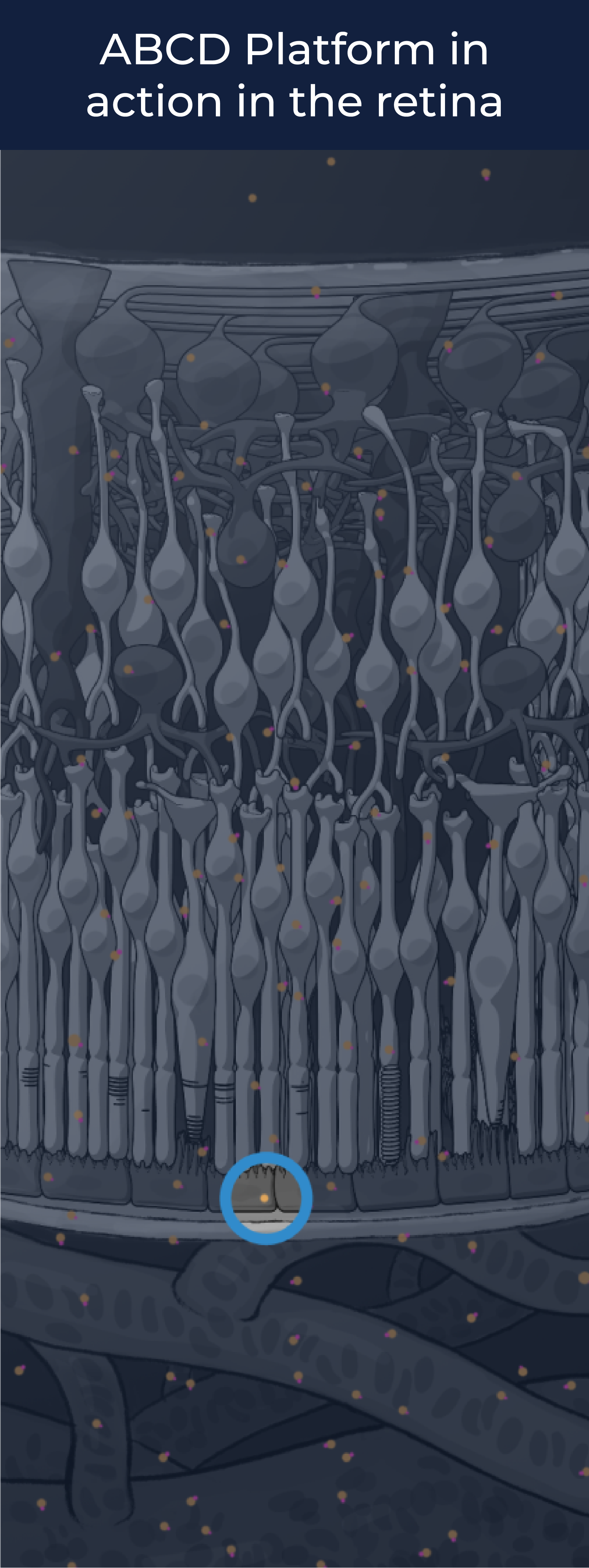



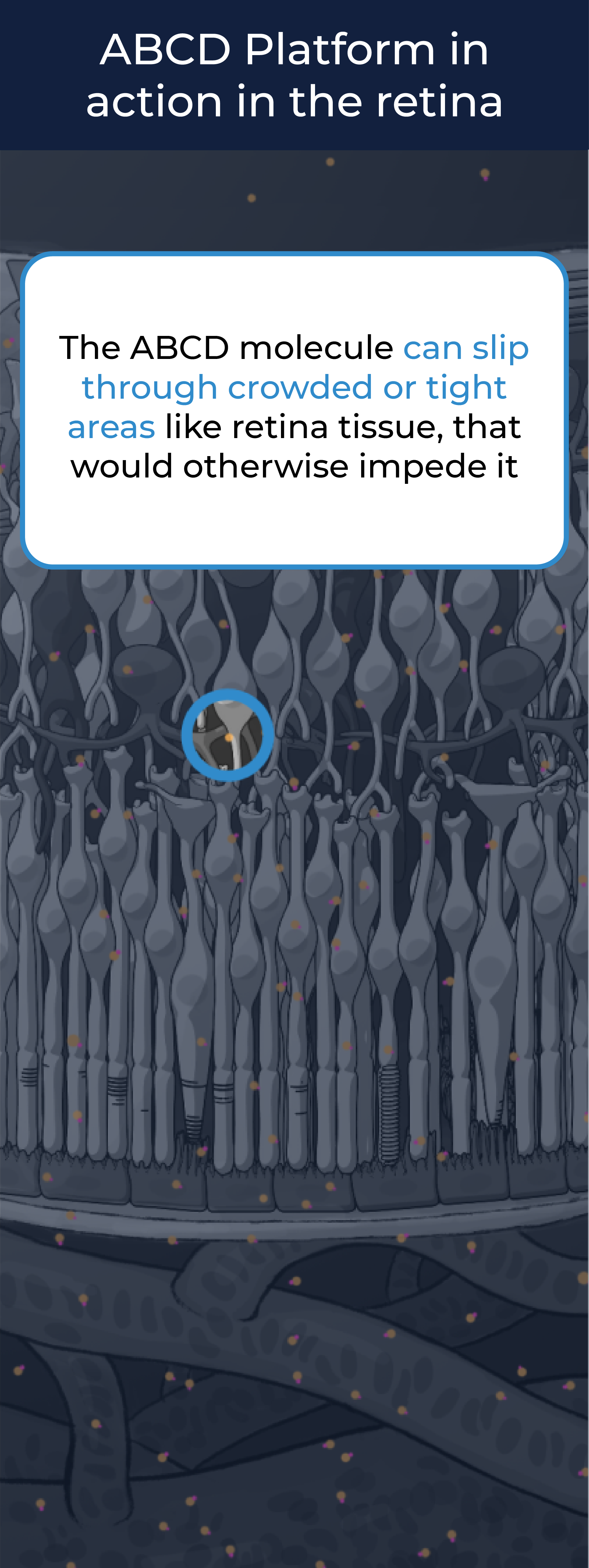
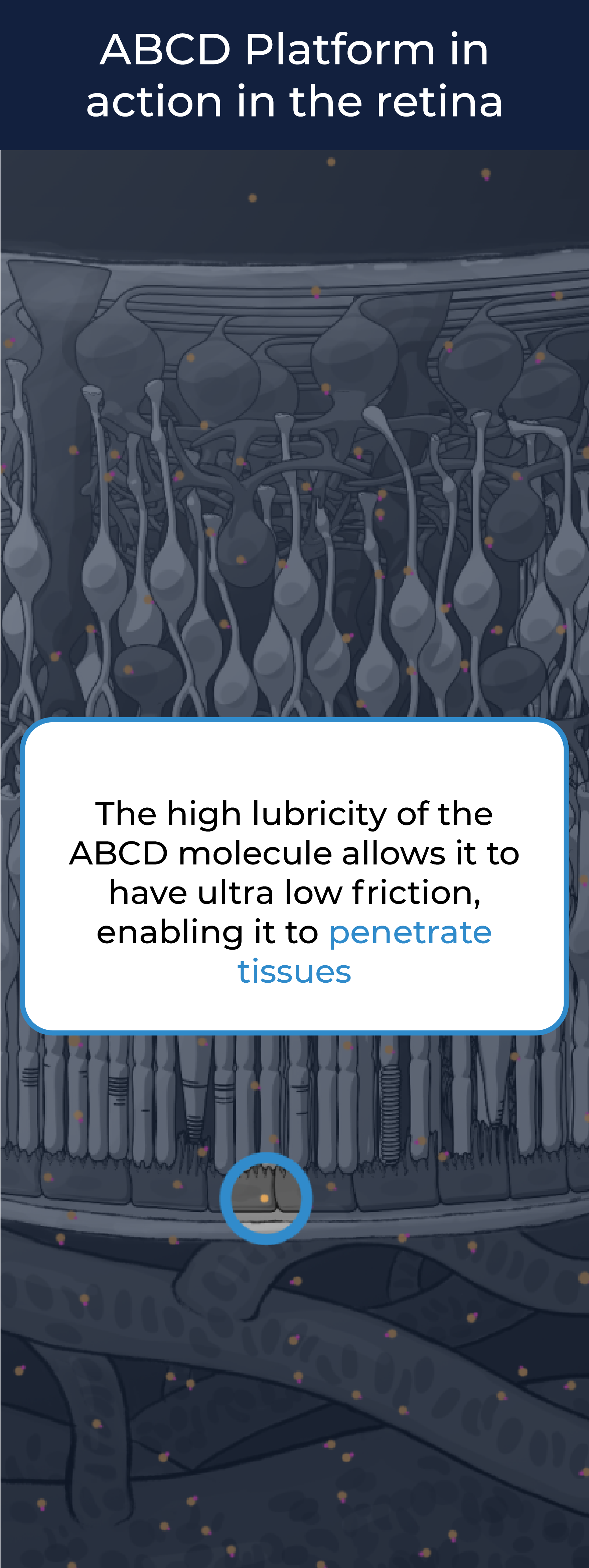

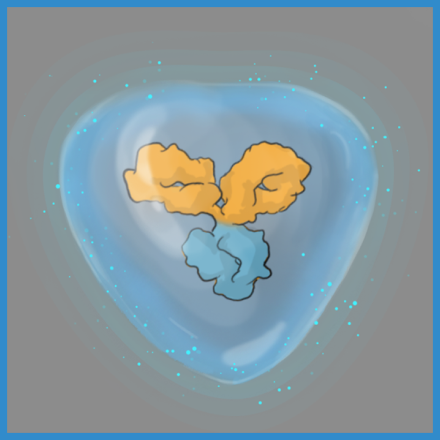
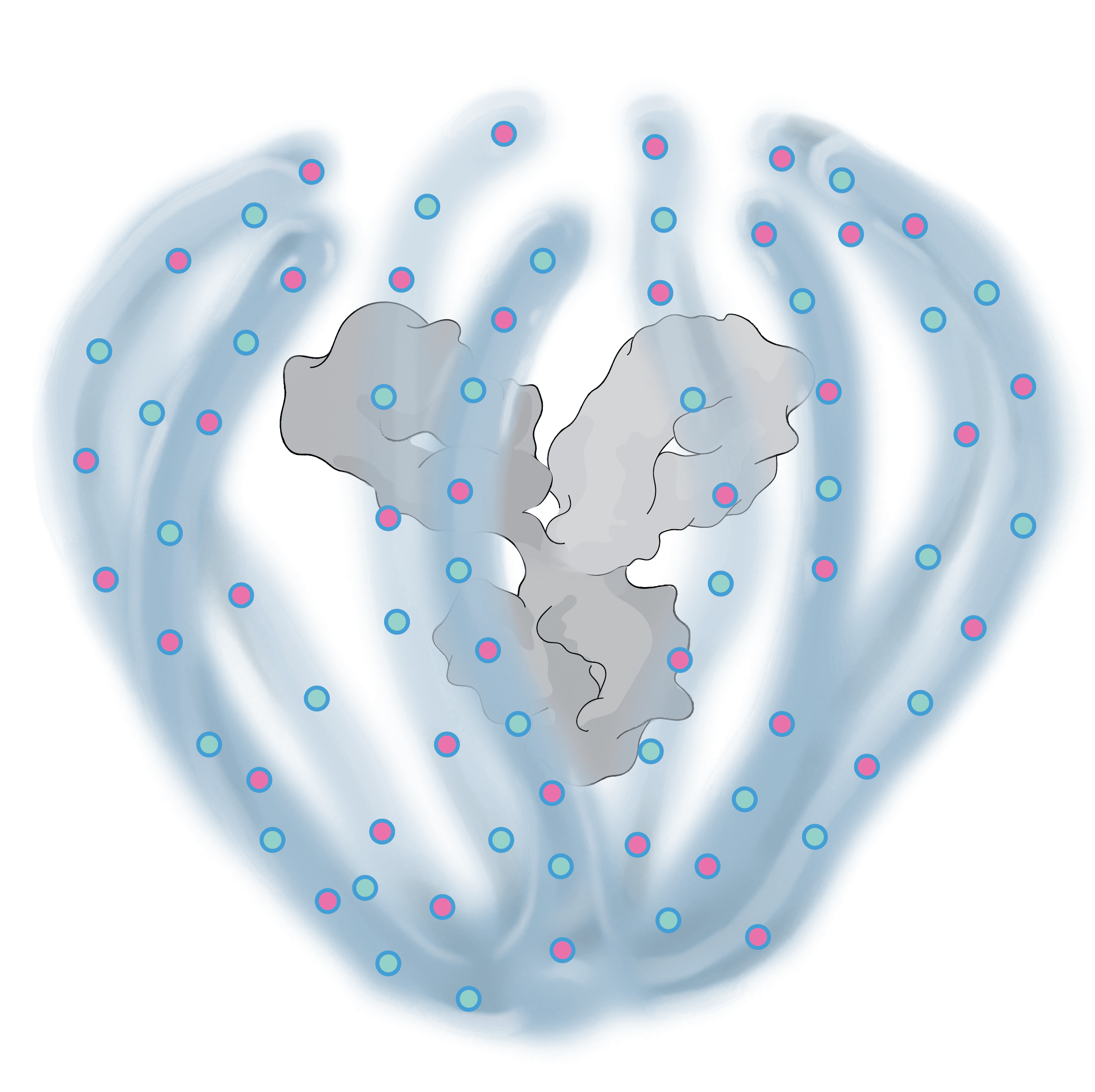
The ABCD Platform uses a bio-inspired polymer to orchestrate water around the antibody without obstructing the binding sites, preventing non-specific interactions
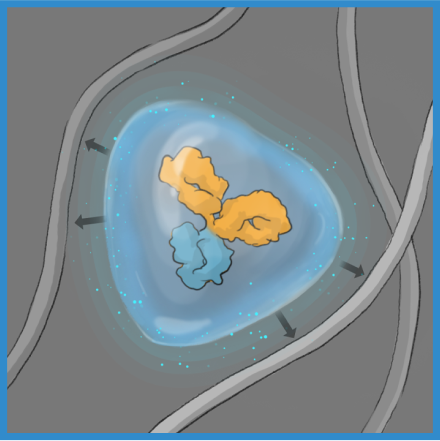
The ABCD molecule can slip through crowded or tight areas like retina tissue, that would otherwise impede it
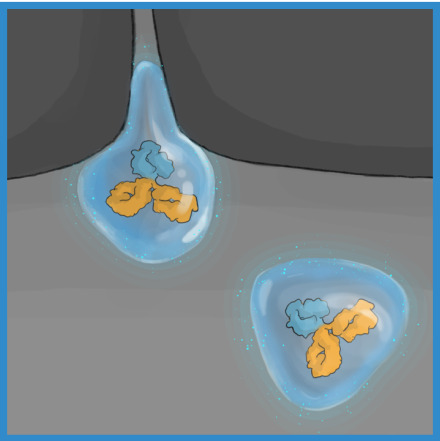
The high lubricity of the ABCD molecule allows it to have ultra low friction, enabling it to penetrate tissues
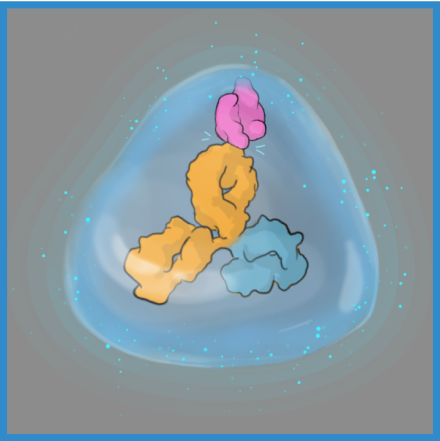
Water influences antibody potency, enabling the ABCD molecule to bind to its target with high affinity and specificity
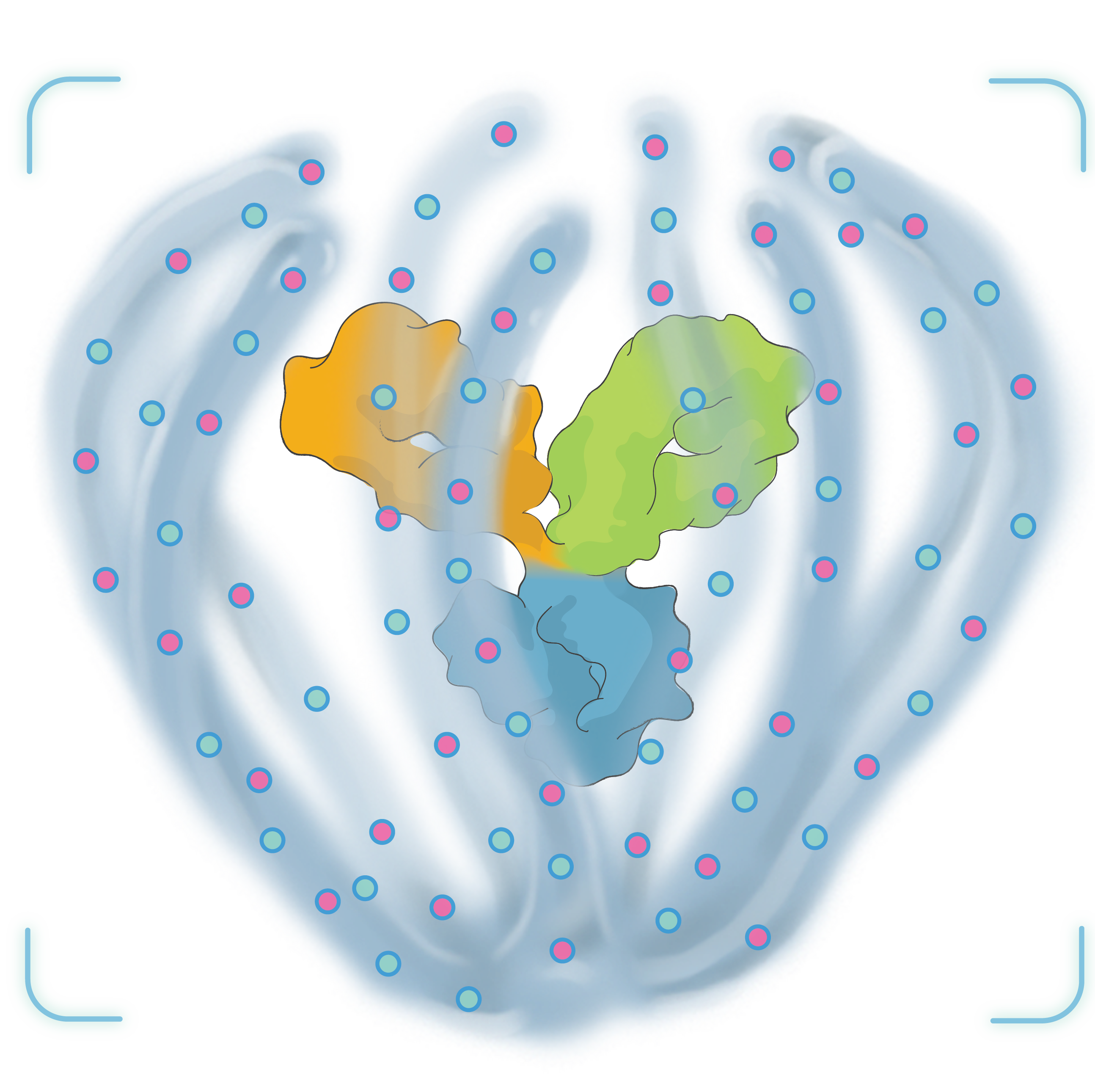
TAKE A 3-DIMENSIONAL TOUR
Tour our Antibody Biopolymer Conjugate (ABC) PlatformTM in 360° below.
Click and drag to see different views. Scroll or pinch to zoom.
Please be patient while the 3-D model loads
Launch the ABC Platform Interactive Experience